Plan & Manage
- Data Management Best Practices
- Create a Data Management Plan
- DMPTool Tutorial
- Sample Data Management Plans
- NIH Policy on Rigor and Reproducibility
- NIH Data Sharing Policy Update
Effective on or after January 25, 2016, most NIH and AHRQ research grant applications are required to comply with the NIH Policy on Rigor and Reproducibility.
For more information, see the official notice:
http://grants.nih.gov/grants/guide/notice-files/NOT-OD-16-011.html
Additional resources that may be helpful to you:
Example Authentication of Key Biological and/or Chemical Resources
Slide presentation of the NIH Rigor and Reproducibility policy (PDF):
These updates will take effect for most research grant applications (including small business and complex research grant applications) submitted for due dates on or after January 25, 2016. For research contracts, this policy will be effective for proposals received on/after January 25, 2016 and expected to result in contract awards in Fiscal Year 2017 and beyond.
For information about the NIH Public Access Policy on published results of NIH funded research, see the UCSD Libguide: http://ucsd.libguides.com/nih.
The requirements for proposals focus on four areas deemed important for enhancing rigor and transparency:
How do I authenticate my key biological and chemical resources?
Reviewers will be asked to consider these additional rigor and transparency criteria when reviewing applications.
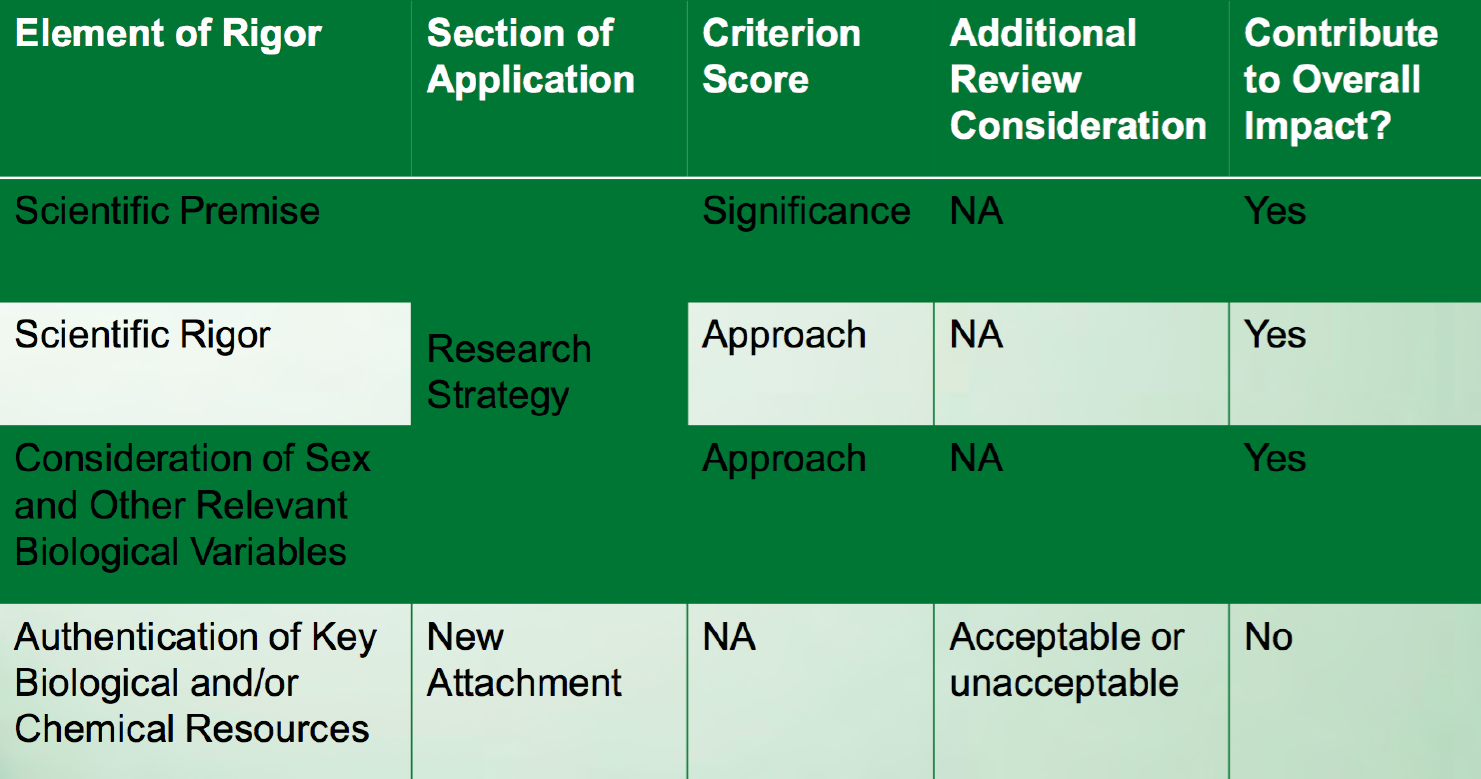
(From Table 12, “Review criteria changes: What will be scored and how will it be scored?” in slide presentation of the NIH Rigor and Reproducibility policy (PDF).)